DSHEA Tutorial: Business Name and Address

While we’re still on the information panel where we’ve listed our ingredients, we’ll need to provide the name and place of business of the manufacturer, packer, or distributor.
You should put that on the information panel which if you don’t remember is located to the right of the principal display panel. The direction of “right” is based on the perspective of the consumer looking at the bottle on a shelf. Now, if your ingredients have filled up that entire information panel, you can continue on to the right on the ingredient panel. Because we’re basically working with a cylinder here, if you move over to the right one more panel, you’re actually on the panel to the left of the principal display panel. Think of it like there’s only three panels. What doesn’t fit on the principle display gets moved over to the right one panel, the information panel. If you run out of space there, you can move to the right one more panel and put the remainder of the information there. However because we’re working on these precut 2″x4″ labels, we’re probably not going to be able to squeeze out three panels, likely just two. Hopefully your product doesn’t have a million ingredients and prevent us from placing this additional information on that information panel.
I’ve seen this interpreted a few different ways. If your business is in the phone book or otherwise easily located all you need is the name of the city, state and zip code. Otherwise, include a complete address. On small labels, I’ve seen an exception whereby only a toll-free number is provided. I also include a website address. While the FDA makes most of these rules to somehow protect the consumer, I find that allowing the consumer to track me down based on the bottle label to be a very important marketing tool. I think of every container as a business card, so don’t skimp on leaving behind bread crumbs so that the consumer can locate you to order more.
Let’s go ahead and add that to our images:
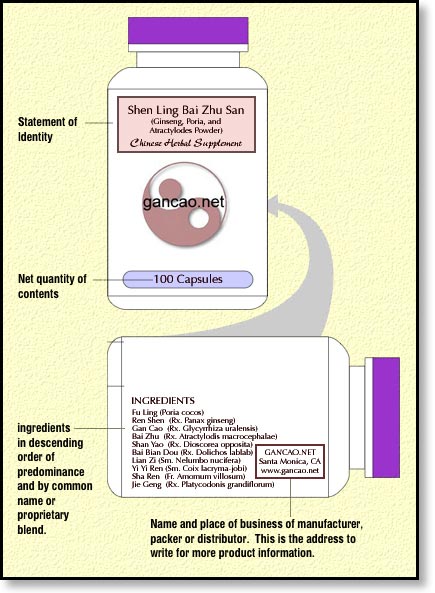
Bottle Label with Name and Address
More recently, another law that deals with the labeling of dietary supplements was passed. This law, called the Dietary Supplement and Nonprescription Drug Consumer Protection Act provides for the FDA to maintain a database of adverse events (“side effects”) of dietary supplements as well as over the counter medications.
The FDA recommends that the label also bear a clear, prominent statement informing consumers that the domestic address or phone number is for reporting serious adverse events associated with use of the product. This language can overlap with the general “contact us” type information on the label. However it must be clearly stated and truthful obviously. As an example, you might write:
To report a serious adverse event or obtain product information, contact. GANCAO.NET, Santa Monica, CA. www.gancao.net.
This applies to all dietary supplements labeled on or after December 22, 2007. Therefore, these labeling requirements are already in effect. However, FDA intends to exercise enforcement discretion for the new labeling requirements until January 1, 2010.
Next: dosage and usage guidelines.
 Last modified: August 25, 2009
Last modified: August 25, 2009  Tags: DSHEA, Labeling В· Posted in: Labeling
Tags: DSHEA, Labeling В· Posted in: Labeling
