DSHEA Tutorial: Statement of Identity

The DSHEA Statement of Identity is essentially the name of the product. For our purposes, we’re going to use Shen Ling Bai Zhu San (Ginseng, Poria, Atractylodes Powder). This is a formula for diarrhea due to Spleen qi deficiency if we just want to give it to our patients. For the general public, we’ll have to water that claim down a bit. More on that when we’re talking about claims.
Let’s begin with our blank labels:
The blank DSHEA label
See how blank it is? Empty cup, blank label. Its like a Buddhism thing. Why don’t we just sit and appreciate its perfection for a moment…
Next, let’s start out with the most basic requirements, in fact I’ll be using the same numbering system as is provided in the source text, just in case you want to follow along at home and cross reference these things. Okay, here we go…
II.1 Statement of Identity
This is essentially the name of the product. For our purposes, we’re going to use Shen Ling Bai Zhu San (Ginseng, Poria, Atractylodes Powder). This is a formula for diarrhea due to Spleen qi deficiency if we just want to give it to our patients. For the general public, we’ll have to water that down a bit. More on that when we’re talking about claims.
The requirements for the statement of identity are the following:
The identity is the common or usual name or an appropriately descriptive term. If you come up with your own formula called “Poop Health” that too would serve as the identity. If it is an unmodified version of a traditional formula, you might consider something like:
Poop Health
(Ginseng, Poria, Actractylodes Powder)
If you modify it you can try something like:
Poop Health
(Shen Ling Bai Zhu San modified)
These parenthetical additions are not required by the FDA, I just suggest it so if one of your patients comes in to my office with your product, I can figure out by the additional information in the parenthesis what it is that this person is taking.
In particular, the FDA states that you must place the statement of identity on the principal display panel. This is a term that takes on more importance as time goes by as everything is oriented off of that principle display panel. Essentially, the principal display panel is the 40% of a round bottle that is visible when the consumer views it on a shelf. Naturally it will contain the most important information such as its name.
Now the FDA also says that you should use bold type face to make the name stand out. The important thing is that it is the first place that the eye goes on the label. They want the name of the product to be clear and obvious. Using an actual bold type face is optional, the important thing is that it is clear and obvious what the statement of identity is. I believe using bold type is optional because in the world of desktop publishing, there is little consistency between a regular type and its bold version.
The official party line on this is: You must make the statement of identity one of the most important features on the principal display panel. To do this, you must use bold type and a type size reasonably related to the most prominent printed matter on the front panel of your label.
Also, the name is supposed to be roughly parallel to the base of the bottle. That’s also just good consumer design. It should be readable when sitting in its normal upright condition. So, don’t get too wacky with angles.
Because we’re going to be dealing mostly with Chinese herbal formulas in this tutorial, we need to add one more issue in regards to the name of the formula. If we’re going to use the pinyin words as the statement of identity, we’re going to have to add in parenthesis the English equivalent. So, we’ll have to start out with something like this:
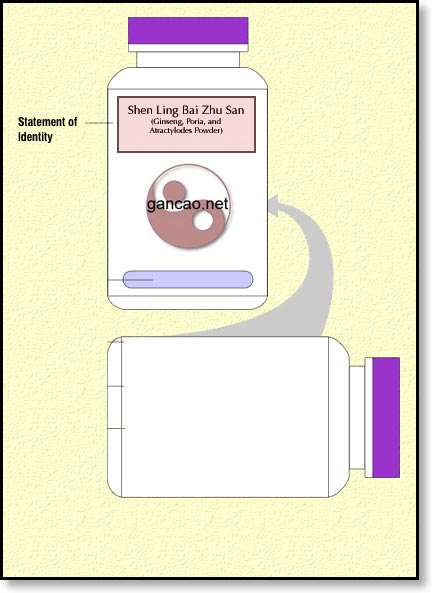
Bottle Label with Statement of Identity
Next: what is this, chopped liver?
 Last modified: August 30, 2009
Last modified: August 30, 2009  Tags: DSHEA, Labeling В· Posted in: Labeling
Tags: DSHEA, Labeling В· Posted in: Labeling
